Conducting Patient and Public Involvement and Engagement activities
Contents
Do you need HREC review for your patient and public involvement and engagement (PPIE) activity?
The guidance on this page is designed to support ethical practice for Patient and Public Involvement and Engagement (PPIE) (see footnote) activities in relation to research and research-related knowledge exchange. We offer principles for all PPIE activity, followed by advice as to whether PPIE activity plans should come through The Open University (OU) Human Research Ethics Committee (HREC) for formal review to gain a favourable opinion for your plans. We conclude with examples of PPIE activities which could be involved in a project from its design to dissemination, to illustrate recommended practice for ethical review.
Although this guidance is primarily for research-related PPIE activity, you may find it useful to consider the principles below when involving members of the public in OU teaching.
PPIE ethical practice principles
- Clear communication to public contributors which allows you to feel confidence in gaining their informed consent to be involved in PPIE activity.
- PPIE contributors should be clear about what they are being asked to do or to contribute, their role, the schedule, and any payment/expenses that will be offered.
- Refer to PPIE guidance for the payment of public contributors (internal link only) to help you assess whether a public contributor’s role means they are a volunteer or a limited engagement or self-employed worker.
- If the public contributor is a volunteer, provide them with an Information Sheet and Consent Form.
- If the public contributor is a limited engagement or self-employed worker and is being issued a consultancy contract, they do not require an information sheet or consent form but it remains best practice to clearly explain the scope of the task they are agreeing to undertake before the contract is sent.
- Consider issues related to equitable recruitment and representation.
- Ensure clarity as to whether public contributors are being asked to represent an organisation or to express their personal views.
- Ensure safeguarding and distress protocols (internal link only) will be in place for PPIE activities related to sensitive topics (see note 1), or with the potential to trigger potential distress.
- Clearly explain to those involved how their input to the activity/project will be used.
- Where the University is acting as the data controller for personal and/or special category data, respect public contributor rights under the UK GDPR/Data Protection Act (2018). This will involve providing public contributors with explanations as to how The Open University will use and store their personal data, and their rights to access this.
- Offer safe spaces for PPIE, which offer public contributors the choice for their contributions to remain confidential or to be recognised. It should be agreed with public contributors how they want to be recognised.
- Where the project creates any intellectual property (IP), provide a clear explanation of what constitutes IP and clarify that this will belong to the University.
- Consider and agree appropriate levels of remuneration for involvement in PPIE activities. See PPIE guidance for the payment of public contributors (internal link only) for more details on payment.
PPIE and ethical review
In the context of research, and when appropriate, PPIE should be integrated from design to dissemination. This inclusion will ensure that the appropriate approvals have been completed for all research and data collection, and avoid delays caused by the need for additional reviews.
The following teams are involved in OU approvals in relation to research:
- The Data Protection team (internal link only) – for any personal and/or special category data (see note 2)
- The Library Research Support team – for research data management planning (see note 3)
- Information Security (internal link only) – for any tools used to gather data, not already covered for use
- Student Research Project Panel (internal link only) – for any activities related to current OU students about their learning experience
- Staff Survey Project Panel (internal link only) – for any activities approaching over 30 members of OU staff
- The Human Research Ethics Committee – to offer support, advice on ethical issues and a favourable opinion contingent on the other teams’ approval for your plans
Notes
- Sensitive topics: topics which could illicit distress, put the participants at risk if identified, or cause reputational harm. Examples include: mental health, violence, stigmatised health conditions, discrimination, political or religious beliefs. These topics will require ethical review to ensure that a risk assessment is in place especially when addressing confidentiality and disclosure issues.
- Please note that any audio recordings of discussions must only occur after gaining explicit consent from all participants, and the recordings must be kept secure, confidential, and be transcribed/anonymised as soon as possible and the original recordings then destroyed.
- If you are collecting data for PPIE activities as part of a research project which is considered to need ethics review, a research data management plan is mandatory. This should cover the gathering, storage and disposal of all data, using guidance from the library research support team. These plans are highly recommended for PPIE activities. Research data management plans should be hosted on ORDO as a record of your study.
The above teams may be relevant to projects which include PPIE activities. Whilst it is advisable to anticipate and include PPIE activities at an early stage of ethical approval, applications relating to support for PPIE activities can be made to HREC at any stage in the development of a project.
Where PPIE was included in the original plan and the project has already been through HREC review and there have been changes to plans, including additional activities, you are encouraged to submit an amendment to your original application. HREC commits to providing feedback on amendments within seven working days.
See guidance on how to re-open an application and submit an amendment for review
Where PPIE was not included in the original project plan the guidance below will take you through a set of questions to establish whether ethics approval is required for this work. Most activities will involve ‘low risk’ HREC review and feedback will be provided on these applications within seven working days.
Should the PPIE activities be more complex and evaluated within the HREC application system as ‘high risk’, full review by an internal and external member of the HREC will be conducted to offer support and advice about your plans. You will gain feedback from a full review within three weeks of submitting your application. Coming through the HREC allows your PPIE activities to be covered by a favourable opinion, which can be referred to as part of your PPIE documentation and publications.
Do you need HREC review for your PPIE activity flowchart
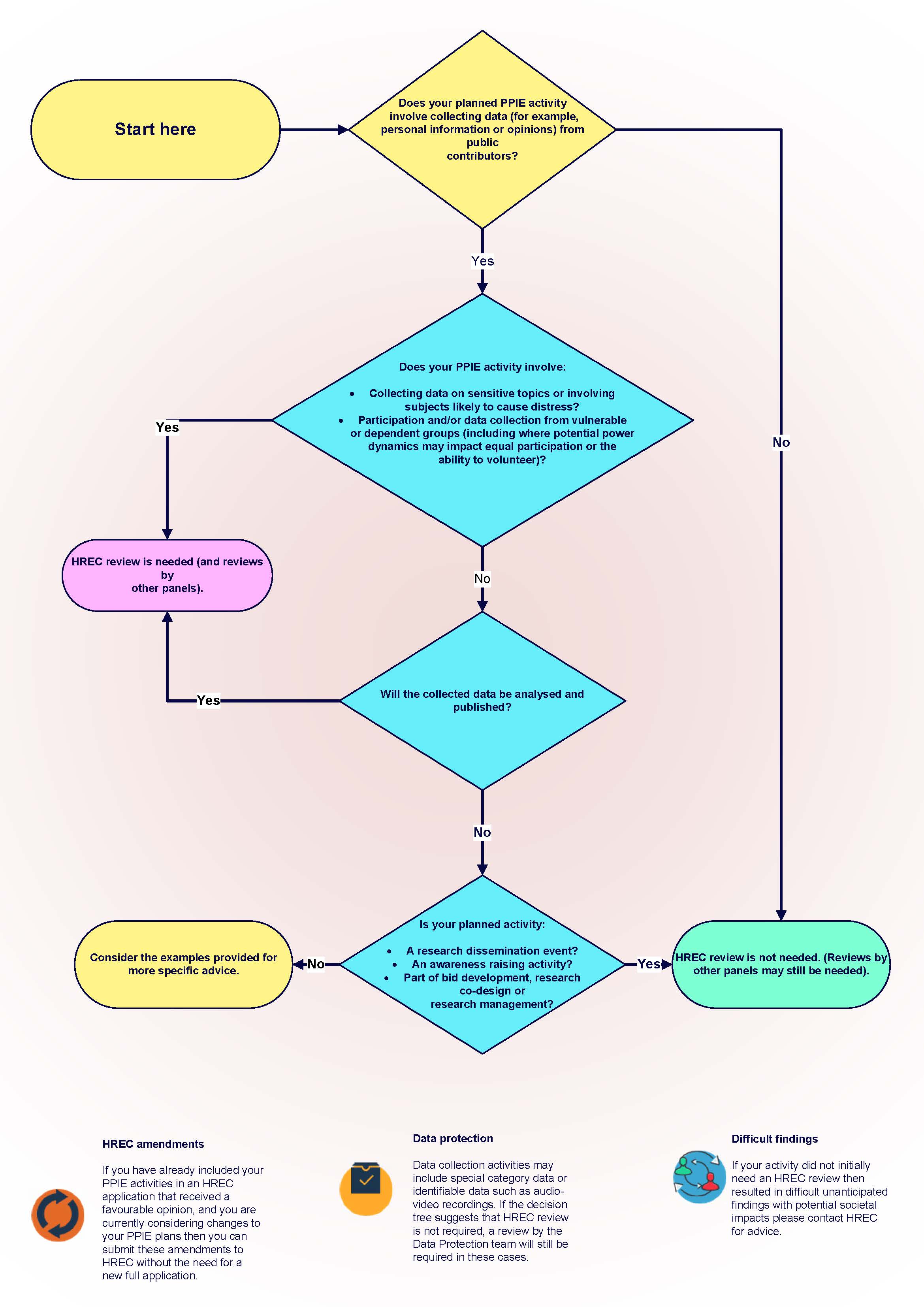
Question 1
Does your planned PPIE activity involve collecting data (for example personal information or opinions) from public contributors?
YES: HREC review could be needed. Consider Q2.
NO: HREC review is not needed but ethical principles should be applied to all PPIE activities.
Question 2
Does your PPIE activity involve:
- Collecting data on sensitive topics or involving subjects likely to cause distress?
- Participation and/or data collection from vulnerable** or dependent groups (including where potential power dynamics may impact equal participation or the ability to volunteer)?
YES: HREC review will be needed, along with review by other teams.
NO: Consider Q3.
Question 3
Will the collected data be analysed and published?
YES: HREC review will be needed, along with review by other teams.
NO: Consider Q4.
Question 4
Is your planned activity:
a) Part of bid development, research co-design or research management?
b) An awareness raising activity?
c) A research dissemination event?
YES: HREC review will not be needed.
NO: Consider examples below for more specific advice.
Examples of PPIE activities
The examples below represent PPIE activities and provide guidance on where ethics approval may or may not be required for this work. Please note this is not an exhaustive list.
Who
Members of the public, people with lived experience, patient support groups.
What
Informing the development of research questions and/or research design methods. This can involve people talking about their own experiences and perspectives. Public contributors might provide comments on draft summaries about the project that are aimed at a non-academic audience (i.e. Plain English Summaries).
How
Usually in meetings/workshops (online or in-person). Could be one-off or a series of meetings. May be accompanied by a family member or carer. All meetings are voluntary and could be individual or group based. Feedback on Plain English Summaries either by email, meetings or telephone. Could also be via surveys to understand a particular experience or to gather views on a research idea. Survey responses are used to further develop the research idea.
Why
Requirement of most funding organisations. Enhances the relevance and feasibility of the research.
Recommended practice
Ethical review or a favourable opinion by HREC may be required (see questions above). If HREC review is not required, please note that HREC is well placed to offer the support needed for researchers to ensure ethical approaches are taken for these kinds of activities. Review the PPIE ethical practice principles for further guidance.
Who
Members of the public, people with lived experience, patient support groups.
What
Lay member of a research study Advisory/Steering committee or a member of the research study PPIE group. Provides a non-academic perspective to the work. Provides comments on the readability of participant facing documents like study information sheets and interview topic guides.
How
Usually in meetings (online or in-person). Could be one-off or a series of meetings. May be accompanied by a family member or carer. All meetings are voluntary. Feedback on documents can be collected by email, telephone, or via meetings (online or in person). Contributions can be at any stage of the research study process.
Why
Requirement of most funding organisations. Enhances the relevance and feasibility of the research.
Recommended practice
Ethical review or favourable opinion may be required. Clear participant information and consent will be important, including clarification of the lay member’s role and forewarning of issues to be covered. Issues of equitable recruitment will be important to consider. Review the PPIE ethical practice principles for further guidance.
Who
Members of the public, people with lived experience, patient support groups, members of communities.
What
Lay co-applicant or a lay co-researcher on a research study (internally or externally funded), i.e. the public contributor is a named member of the research team.
How
Involved in research team meetings, conducting and/or providing feedback into the research design, data collection, data analysis or research dissemination activities.
Why
Including a lay co-applicant is a requirement of some funding programmes such as the National Institute for Health and Care Research (NIHR). Helps reduce barriers to accessing underrepresented communities, which is important for equality and diversity.
Recommended practice
The research study will require ethical review and HREC favourable opinion, and the PPIE activities would be covered within this. Data handling responsibilities need to be agreed as part of the contract and cleared with the data protection team. Particularly important to consider from the outset whether the research team are interested in researching the co-design process, in which case this would need to be agreed with all (including the co-applicant/researcher) as to what counts as data, inviting consent from one another and data protection responsibilities. Review the PPIE ethical practice principles for further guidance.
Who
Members of the public, people with lived experience, patient support groups, members of communities.
What
Sharing research findings through online or in-person events, such as conferences or webinars.
How
Members of the public/patients sharing their experiences of the research findings or attending the event to listen.
Why
Disseminating research findings is an important phase in the research cycle and can lead to impact.
Recommended practice
Ethical review and favourable opinion may be needed. This will depend upon whether dissemination activities are one/off or repeated. The type of data gathered at these events (where relevant) will be an important consideration, along with commitments to anonymisation and deidentification. Consideration should be given to how representative/diverse/inclusive the event is. If this is considered as a research project extension and the data gathered will be included and reported with the research, then HREC review, and a favourable opinion will be needed. It is always preferable for these types of activities to be built into the original application where possible. Review the PPIE ethical practice principles for further guidance and please seek advice from HREC as to whether an application is required.
Who
Members of the public, people with lived experience, patient support groups, members of communities.
What
Sharing their ‘lived experience’ to inform and/or co-create teaching and learning material.
How
Participating in an interview about their experience to inform case study material and providing feedback on draft teaching material related to their own or the ‘service user’ experience.
Why
Involving patients and service users in teaching and learning materials is a requirement by some regulatory bodies (e.g. related to social work, or nursing).
Recommended practice
So long as there are no plans to theorise and publish as academic research this would not fall within HREC remit. Targeting those with special category classifications will require data protection team review and approval. The PPIE ethical practice principles may be helpful to review.
Who
Members of the public, people with lived experience, patient support groups, members of communities, charities, community groups, professional organisations.
What
Two-way process where academics, professionals, organisations, members of the public or service users share learning, ideas and experiences.
How
Participating in meetings, workshops, Evidence Cafes. Also includes community-led projects where the OU is providing funding, advice and support.
Why: Pathway to delivering benefits to the wider society. Activities may contribute to impact case study development, and the engagement/environment statement in REF, and also to the Knowledge Exchange Framework (KEF) framework.
Recommended practice
Ethical review and a favourable opinion may be required, and it would be advisable to seek advice from HREC in the first instance. If theorised and published as academic research this would fall within HREC remit for review and a favourable opinion. Review the PPIE ethical practice principles for further guidance.
Footnote
Patient and Public Involvement and Engagement (PPIE) is embedded across the OU in our research, teaching and learning, knowledge exchange, impact and Open Societal Challenges activities. For this guidance, we define PPIE as activities that are carried out ‘with’ or ‘by’ members of the public (‘public contributors’). PPIE is also used in co-production/co-design, where the public work together and share power and responsibilities with researchers and stakeholders, from the start to end of the project. PPIE is different to research participation, as the latter refers to research that is done ‘to', 'for' or 'about' patients and the public.