Human Research Ethics in Undergraduate and Taught Postgraduate student projects
The purpose of this page is to offer guidance to Open University module teams on how to implement ethical oversight of student research projects that involve human participants. This includes projects that recruit and collect data directly from human participants, including students (e.g., conducting interviews, focus groups, surveys, experiments), and projects that gather data provided indirectly by human participants (e.g., harvesting social media posts, making undisclosed observations).
Suggested broad protocols for ethics review are offered, and some examples of documents and templates that could be adapted to a module team’s specific needs are offered.
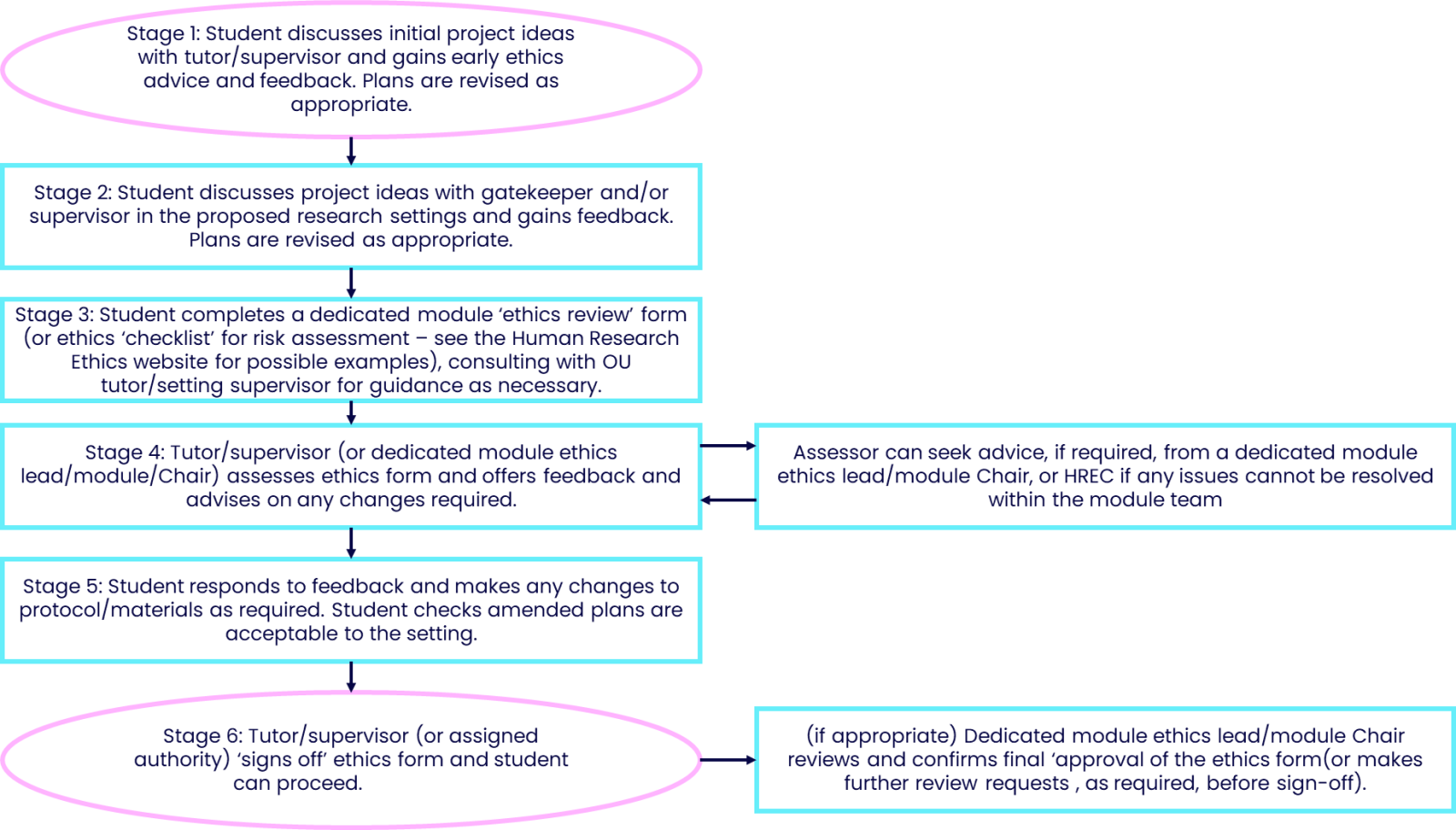
Ethics review of student projects – suggested process (to be tailored by module teams as appropriate)
Stage 1
Student-tutor consultation: Initial discussions and advice regarding the research project work will take place in different ways and at different stages depending on the specific module requirements. Discussing any ethics issues and concerns early on is advisable, so as to pre-empt any subsequent problems that were unanticipated earlier.
Stage 2
Student-setting consultation: For reasons both of any required additional (eg workplace) insurance cover and ensuring that the research is accepted and supported in a research setting, early negotiations with a gatekeeper and/or delegated supervisor can establish that the research can take place and also that support will be offered throughout the study. Feedback including any concerns should be incorporated into the research design.
Stage 3
Complete ethics review form: It is advised that module teams require students to submit an ‘ethics review’ form (or suitable alternative, e.g., an ethics risk assessment checklist) that will be assessed by an appropriate designated reviewer. This may be the student’s tutor, a designated module team ethics advisor, or the module Chair, for example. Please refer to the Human Research Ethics website for a template ethics risk assessment checklist, which can be adapted, elaborated, expanded as appropriate to the specific context and module requirements.
Participation Information Sheets and Consent forms: Students should be asked to submit Participant Information Sheets (PIS) and Consent forms with their application if they are going to be directly recruiting participants. Copies of the standard HREC PIS and Consent templates that are provided for use by Open University researchers can be found on the Human Research Ethics website.
Note: It may be appropriate for module teams to simplify these templates, for example removing some of the higher risk elements (e.g., working with children, discussing sensitive topics), depending on the specific context and module requirements for student projects. However, certain key elements are normally expected to appear in a PIS and Consent form – see Appendix 1 for advice on PIS and Consent forms for module teams. If you would be interested in seeing how these templates have been adapted into ethical protocols, participant information sheets and consent forms for different kinds of research task, please contact the WELS ECYS Masters email box putting EE831 and E822 ethical documentation query in the subject line.
Stage 4
Assessment of Ethics Review Form and feedback: A suitable person on the module team (e.g., tutor, research project ethics lead) should assess the ethics form and raise any concerns as constructive feedback, clearly explaining any issues and making recommendations for how these can be resolved.
Stage 5
Student responds by submitting updated Ethics Review Form: The resubmitted Ethics Review Form should incorporate all advised changes in order to resolve any ethics concerns. This may involve adapting the original design of the study and may also involve some modifications to the participant-facing documentation (PIS and Consent forms, study materials, e.g., survey, interview schedule, etc.). These amends should also be cleared by those in the research setting supporting the research.
Stage 6
Re-assessment and final sign-off: Assuming advice has been properly taken on board, and any ethics concerns raised resolved, the Ethics Review Form can be given final ‘sign-off’. The student can then proceed with their research project. Some module teams may wish to involve two levels of sign-off, e.g., first by the student’s own tutor, and then by a dedicated ethics lead on the module team. Processes may differ depending on the individual module requirements and constraints.
Additional considerations and resources
Module teams, tutors and students should be aware that cover by University insurance policies for research will apply also to Undergraduate (UG) and Taught Postgraduate (TPG) student projects ON THE UNDERSTANDING that projects have been fully risk assessed and approved by the Faculty before commencement, according to module/Faculty policy. This means it is important for module teams to be able to demonstrate that risk assessment and approval processes and protocols are in place for all student project work. Student UG/TPG projects are NOT assessed by the Human Research Ethics Committee (HREC) and do not require HREC review. Any incident leading to a claim will be assessed according to whether proper ethics processes have been followed.
This means that students will need to make their own arrangements, and module teams will need to give guidance on when this may be necessary, depending on the context and assessment of risks. If employed in the setting in which the research will be carried out, and students have sought support for their plans from their employer, or another gatekeeper in the research setting, they should clarify whether employer/gatekeeper support offers them insurance cover. It is recommended that a signed documented statement of support for the research is gained from the organisation/setting, that also identifies a gatekeeper/supervisor who will ensure support and logistical advice on the research project while it is conducted. Please refer to the Human Research Ethics website for template documents which can be adapted for these purposes.
The safety and wellbeing of both students and their potential research participants are crucial, so students and/or module teams should consider whether any of the following information and policies are relevant to what the research project work entails. If so, it may be appropriate to refer explicitly to these policies on the ethics review form and ask for further information on these aspects. However, module teams might find it best to ensure students avoid these higher risk elements, unless absolutely necessary. Where higher-risk projects are required, identifying a gatekeeper (e.g., employer) who would be able to oversee plans and potentially offer liability cover, may offer an effective extra safeguard measure. Please see the Human Research Ethics website for template documents that could be adapted in order to approach gatekeepers.
If students will be alone with a research participant the Lone working policy (internal link only) will apply: (lone workers are defined as those who work by themselves without close or direct supervision).
Students who will be in contact with vulnerable adults or children or the study might lead to disclosures of potential harm or violence should familiarise themselves with the Safeguarding information (internal link only).
If required an appropriate level of disclosure (‘police check’) can be obtained from the Disclosure and Barring Service (England and Wales), Disclosure Scotland, Access NI (Northern Ireland), Criminal Records Office (Republic of Ireland). Details can be obtained on the OU’s Arrange a DBS check intranet pages (internal link only).
The Health Research Authority (HRA) has recently introduced new eligibility criteria for standalone NHS based student research. Some master’s level students will be able to apply for ethics review and Health Research Authority and Health and Care Research Wales (HCRW) Approval or devolved administration equivalent. Standalone research at undergraduate level that requires ethics review and/or HRA and HCRW Approval (or devolved administration equivalent) cannot take place. Please see the HRA website for further details about the new eligibility criteria.
It is an offence in UK law to view or otherwise access via the internet documents or records containing information likely to be useful to a person committing or preparing an act of terrorism. There is an allowable defence if the information/material being viewed or downloaded is being used for approved, academic research purposes. Module teams wishing to support their students to undertake independent research involving the collection, recording, possession or viewing on the internet of security-sensitive research materials, relating to terrorism, extremism or radicalisation must ensure that they have processes in place to support students to adhere to the Taught Students Terrorism and Extremism-Related Research policy. Further information, guidance and resources can be found on the Taught students terrorism and extremism-related research intranet page (internal link only).
Module teams (particularly where a significant number of students will be undertaking research projects involving human participants) are strongly advised to designate an expert ethics advisor who can be contacted by tutors/reviewers, if necessary, regarding any projects that raise particular ethics difficulties than cannot be resolved during the review process (e.g., module team ethics lead, programme lead, school lead, etc.).
Where further advice on ethics issues is needed, then consulting a faculty lead (if available), or at the highest level – the Human Research Ethics Committee – for further advice may be appropriate. For example, if unforeseen ethics risks or problems occur during a project, then this level of consultation may be appropriate for urgently required advice.
Guidance for module teams on Participation information and Consent
Persons from whom data are gathered should have consented freely and voluntarily to participate, having been given sufficient information to enable them to make an informed choice. They should be free to withdraw or modify their consent and to ask for the destruction of all or part of the data that they have contributed within agreed and consented limits. Consent can only be legally gained from those over the age of consent for that particular country and for those who are deemed able to give informed consent. For children and young adults up to the age of 17 (if in the UK) and those considered vulnerable, whilst consent should be sought from guardians, then the individuals’ assent should also be actively sought after explaining the research requests of them in ways appropriate to them.
Consent is not valid unless it is given from an informed perspective. Giving potential participants necessary and sufficient information about what they are being asked to contribute, and why, in an understandable form is crucial to giving them an adequate basis for deciding whether or not participate. This requires careful thought about the most appropriate means to use, which might include oral, pictorial, audio, or video media as well as or instead of a textual information sheet.
The following list offers a series of headings for consideration. Not all of these will be relevant in specific cases.
- The aim(s) of the data gathering.
- The type(s) of data to be collected.
- The method(s) of collecting data.
- Confidentiality and anonymity conditions associated with the data including any exceptions to confidentiality, for example, with respect to potential disclosures.
- Compliance with the Data Protection Act (2018).
- The time commitment expected from participants.
- The right to decline to offer any particular information requested.
- The opportunity to withdraw from the study at any time during participation, and up until a certain date post-participation, e.g. after data have been anonymised or aggregated, with no adverse consequences.
- The opportunity to have any supplied data destroyed on request (up to a specified date).
- Details of any risks associated with participation.
- The name and contact details of the person leading the data gathering.
- The name and contact details of another person who can receive enquiries about any matters which cannot be satisfactorily resolved with the lead person (for example a tutor or module team member).
- Any debriefing that is planned and how the results of the research will be made available to participants.
- How the data will be owned, stored and used, and future uses including in open datasets.
- A Privacy Notice.