You are here
- Home
- Open Societal Challenges
- Sustainability
- Increasing the sustainability of meat production
Increasing the sustainability of meat production
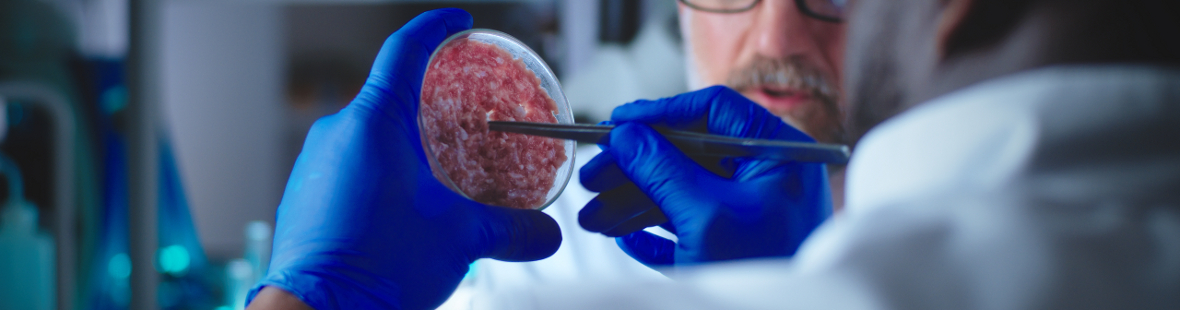
We may consume less than we used to, but meat remains an important British staple: the average Briton eats a significant 84.2kg every year – almost double the global average! Increasing awareness of the problems associated with this high consumption is leading many to consider healthier and more sustainable alternatives, so it's no surprise that recent advances in lab-grown meat have caught the public’s interest. This exciting field is still in its infancy but already Open University researchers are seeing potential far beyond a simple ethical meat substitute.
"We need to increase the sustainability of meat production to reduce emissions," explains Jim Hague, a theoretical physicist at the Open University. "Cultured tissues could also improve the success of pharmaceutical trials while reducing animal testing, and significantly advance regenerative medicine to support those with injuries, illness and old age."
The production process begins with a small sample of cells, painlessly collected from a healthy animal. This sample is mixed in a warm nutrient-rich solution called growth medium and over a number of days, the cells grow and multiply to form a uniform slurry of genuine meat. This meaty mush is perfect for processed foods like burgers and nuggets but higher-quality or medical-grade products require far greater control over the structure and composition of the cultured tissues. Moulds and scaffolds can help provide some of this missing structure, encouraging the cells to organise themselves into part of a larger biological unit such as an organ or muscle. However, gaps in our understanding mean this is an imperfect process and cultured tissues still have a way to go before they can accurately mimic the original.
"Developing tools to control large tissue growth is vital to bring about long-term improvements to cultured meat, drug screening assays and regenerative medicine," says Dr Hague. "The correct organisation of cells is important in all these fields, particularly for regenerative medicine."
Structure and function are closely related so designing the right scaffold is imperative to get a realistic working tissue. It's this problem which Dr Hague, surrounded by a multidisciplinary team of physicists, biologists, and machine learning specialists, hopes to tackle. Supported by the Open University’s Open Societal Challenges programme, the team are working on a number of algorithms and machine learning tools to simulate and predict the properties of tissues grown in a variety of different moulds and scaffolds. Investigating systems from the scale of cells, right through to the dimensions of organs, they hope to develop an effective tool to automatically design the most appropriate growth scaffold for any type of tissue. These designs will then be tested experimentally, using 3D-printed moulds and scaffolds to investigate how closely the simulations match the biology.
"Our initial focus will be using our techniques to design scaffolds for improved cultured meat as this will have the most immediate impact and lead to more sustainable food sources with lower greenhouse gas emissions, while reducing animal suffering," says Dr Hague, "Eventually we would like to develop scaffolds for pharmaceutical assays and regenerative medicine." The team have already developed a number of methods and machine learning tools to predict the organisation of cells in cultured tissues and are planning to mature these techniques further, to reduce computation time and extend the approaches to track moving cells.